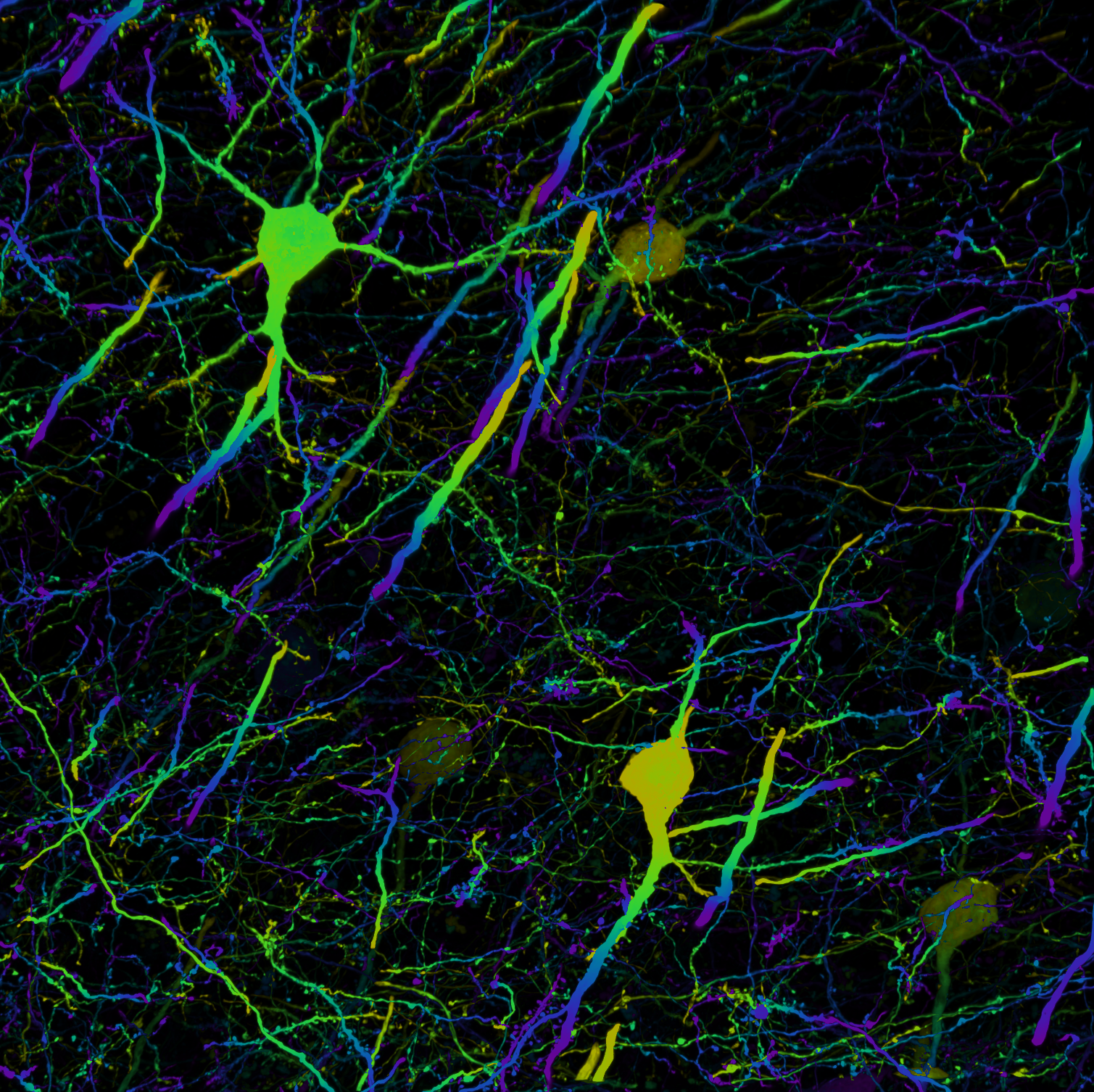
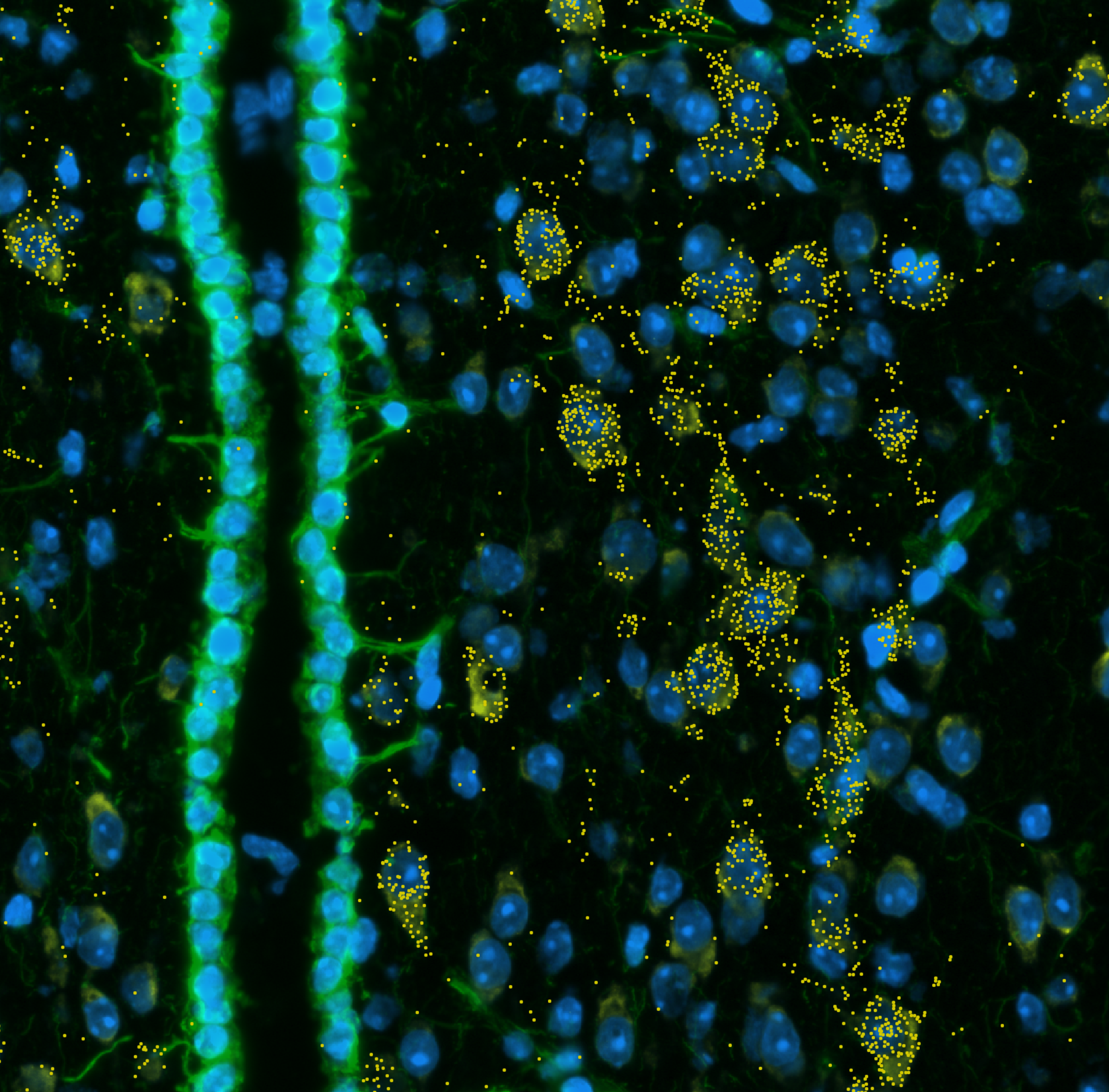
The Microscopy and Flow Cytometry Facility (MFC) provides researchers with advanced tools for light microscopy, flow cytometry and cell sorting, along with access to standard and high-end instruments, technical training, assisted services and expert consultation. As a Nikon Center of Excellence, the facility offers comprehensive service and support in collaboration with Nikon.
Quick links

Getting Started
Step 1: Consultation
MFC offers initial consultations to discuss your samples, experiment goals, and how MFC staff can best support your project. Email us to schedule a consultation.
Step 2: Request Training
Training is required for use of any MFC equipment.
Step 3: Reserve Instruments
Reservations are first-come, first-served with no limits on length or quantity. Log in to the FBS system to reserve instruments after training is complete.
Acknowledgment of Core Services and Equipment
Please acknowledge the use of core services and instruments in your publications. Proper citation directly impacts funding for this facility.
RRID:SCR_021756
If a staff scientist has significantly contributed to a project, please consider acknowledgement or co-authorship.
Contact the Facility Director to request a grant submission letter of support.
Services
MFC offers provides training for independent instrument use, assisted operation by staff scientists, and consultation to identify appropriate tools for research needs. Training is available to UT Austin researchers, with assisted use offered to external academic and industry users.
Training for Independent Use
Training for independent use is only available to internal UT researchers.
MCF provides one-on-one, in-depth training on all instruments to internal UT researchers. Training consists of instrument demonstrations and time for you to practice and learn hands-on. Training is required for unassisted use of all equipment.
After training, you will have access to book time for your independent use of the instrument through FBS. MFC staff will always be available for assistance, troubleshooting, or to help with the setup of a new type of data acquisition.
Assisted Instrument Use
Assisted use of MFC instruments is available to internal and external researchers.
For experiment data without the time commitment of training, assisted use is offered, in which staff scientists will operate an instrument for you.
Please email us to schedule assisted use.
Consultation
We offer initial consultations to discuss your samples, experiment goals, and how our microscopy and flow cytometry services and instrumentation can best support your project.
Please contact the Facility Director to schedule a consultation
External Users
Training is only available to internal UT researchers, but assisted use services is offered to anyone external to UT Austin. UT System personnel can access the facility’s services at internal rates. Non-UT System academics or industry users have a different fee structure.
Instrumentation
MFC offers advanced instruments for data analysis, flow cytometry, and light microscopy. All instruments require mandatory training and usage is reserved in advance through FBS (first-come, first-served). UT Researchers may access the facility after hours upon gaining sufficient experience. However, staff will not be available for assistance during those times.
Data Analysis Workstations
MFC provides dedicated workstations to assist with your image and flow cytometry data analysis needs. Access is available to the following software packages:
- Imaris - Advanced 3D and 4D rendering and quantitative analysis, including particle tracking, cell counting, filament tracing, and machine-learning segmentation. Remote access available.
- Nikon NIS-Elements – Offline analysis software for Nikon microscope images. Features include 3D reconstruction, deconvolution, cell counting, intensity analysis, JOBS automation, spectral unmixing, STORM analysis, and AI-based segmentation. Remote access available.
- Zeiss ZEN – Offline software for Zeiss microscope images, supporting Airyscan reconstruction, 3D rendering, FRAP, image processing, and analysis.
- FlowJo – Comprehensive flow cytometry analysis platform for data visualization, gating, compensation, and single-cell analysis.
- FCS Express – Flow cytometry analysis software with robust data visualization tools and publication-quality graphics.
MFC offers bulk licensing to FlowJo at a discounted rate through an annual seat purchase program, allowing users to install FlowJo in their own labs. If you would like to purchase seats, please contact the Facility Director.
Each user is responsible for long-term data storage. The MFC will regularly delete data from workstations when space is needed, and experiment data is not backed up.
Flow Cytometry
Flow cytometry is a technology that analyzes multiple physical and/or chemical characteristics of single particles, usually cells, as they flow in a fluid stream through a beam of light. The properties measured include a particle’s relative size, relative granularity or internal complexity, and relative fluorescence intensity.
Light Microscopy
Light microscopy allows the visualization and analysis of the structure and function of biological and material samples. The MFC has a suite of microscopes for imaging a wide variety of sample types and at all levels of resolution. Designated a Nikon Center of Excellence, MFC offers advanced instrumentation, comprehensive service, and support in partnership with Nikon.
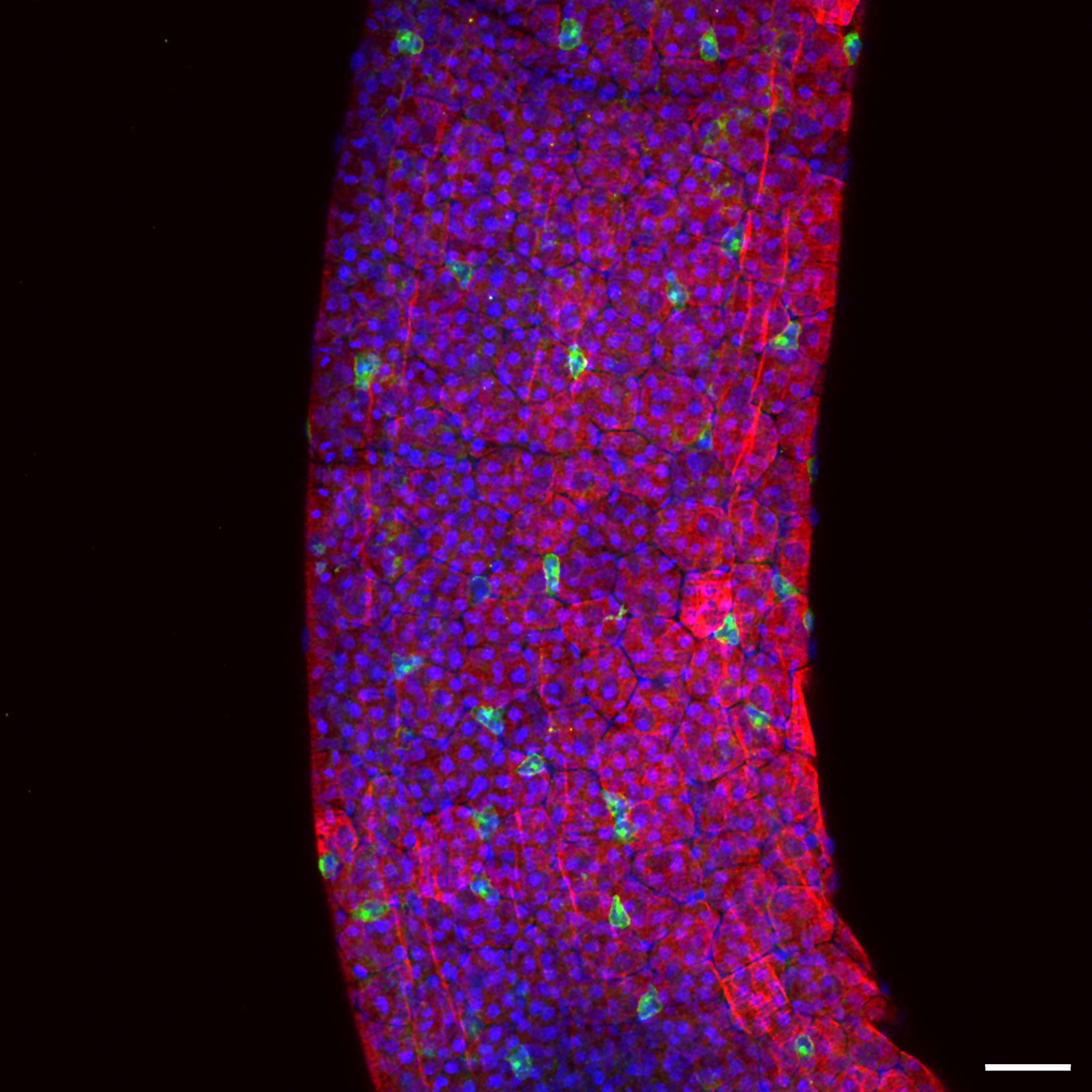
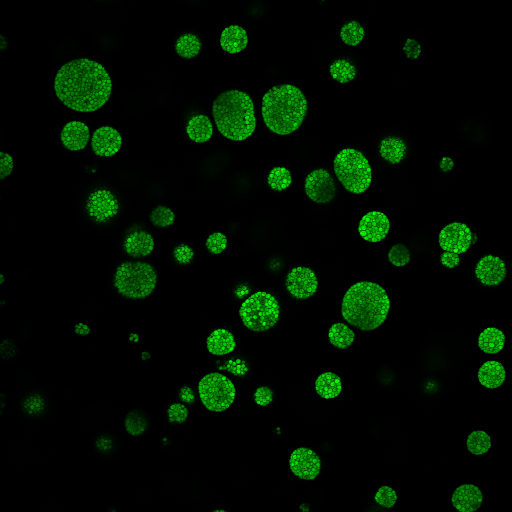
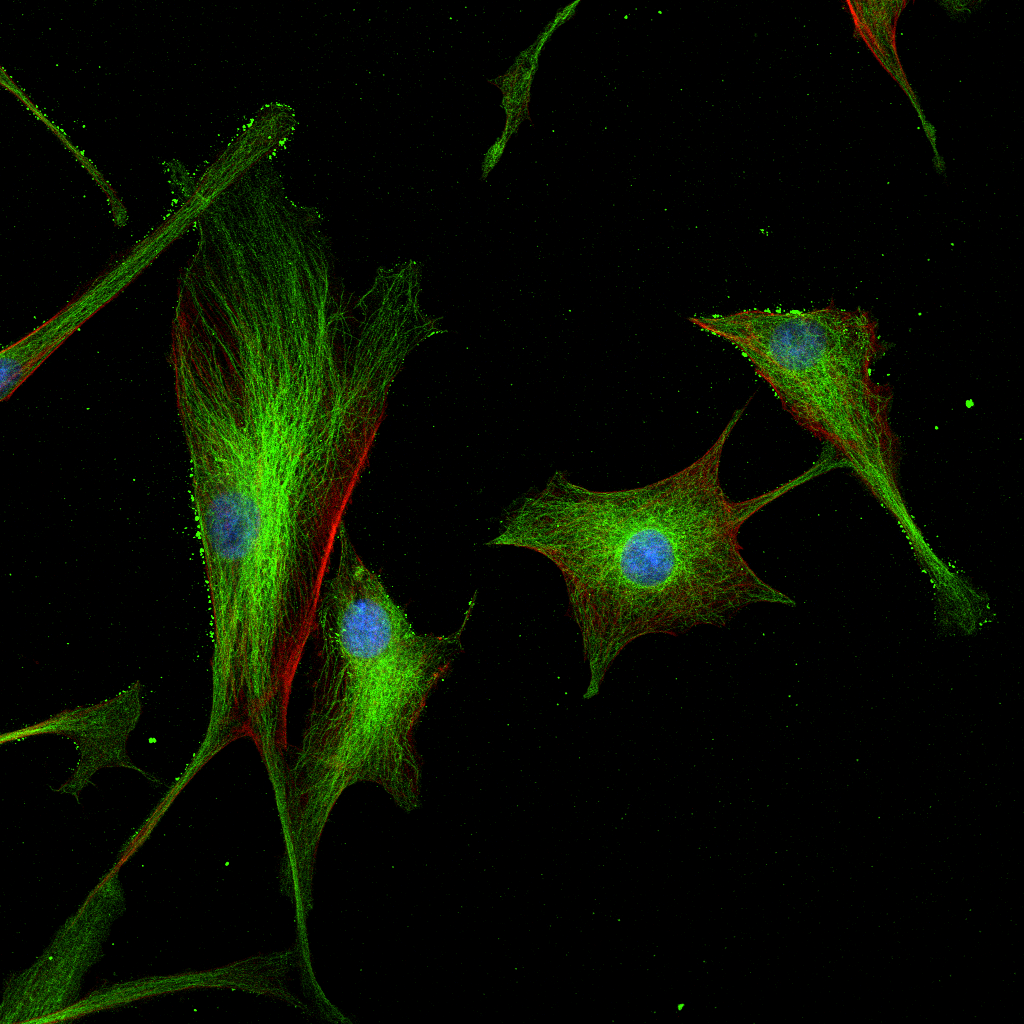
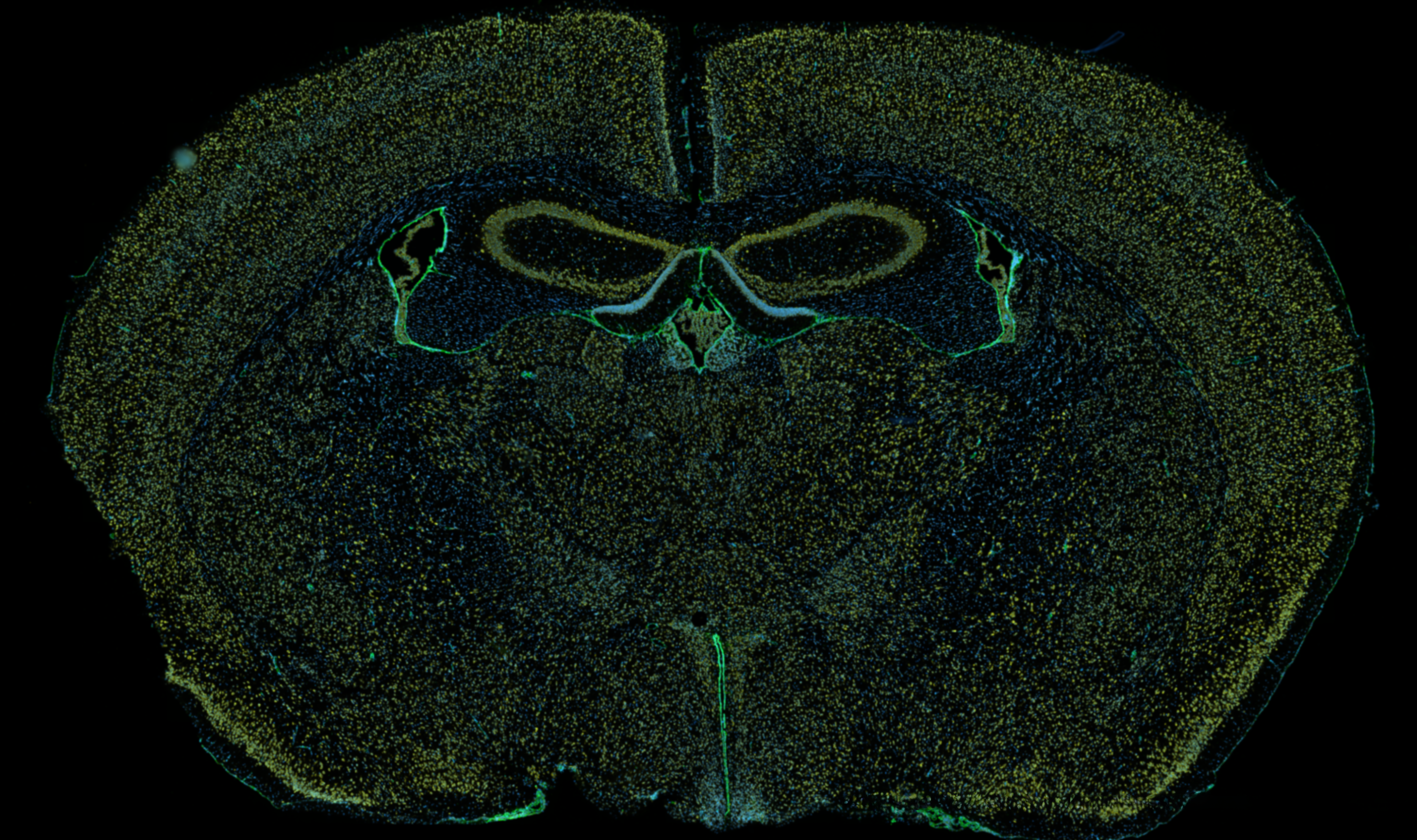
Images captured using advanced microscopy techniques available through the Microscopy & Flow Cytometry Facility.
Additional Resources
Fees
Billing is based on either the reserved time or actual usage—whichever is longer. Standard instrument rates apply to late cancellations and no-shows.
Confocal and Super Resolution Microscopy
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| Nikon AXR Inverted Confocal | $58 | $48 | $113 | $168 |
| Nikon AXR Upright Confocal | $58 | $48 | $113 | $168 |
| Nikon AXR 8-Laser Confocal | $58 | $48 | $113 | $168 |
| Nikon W1 Spinning Disk Confocal Microscope | $58 | $48 | $113 | $168 |
| Nikon NSPARC Super Resolution | $58 | $48 | $113 | $168 |
| Nikon TIRF Microscope | $58 | $48 | $113 | $168 |
| Nikon N-STORM Super Resolution | $58 | $48 | $113 | $168 |
Multiphoton Microscopy
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| Nikon Multiphoton Microscope | $58 | $48 | $113 | $168 |
Compound Microscopy*
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| Nikon Eclipse Ni Compound Microscope | Free | Free | $55 | $110 |
| Nikon Eclipse Ji Fluorescence Microscope | $28 | $24 | $83 | $138 |
| Nikon High Content Analysis Microscope | $28 | $24 | $83 | $138 |
| Nikon Ts2R Inverted Fluorescence Microscope | $28 | $24 | $83 | $138 |
| Nikon Ni-E Upright Fluorescence Microscope | $28 | $24 | $83 | $138 |
*Our longitudinal imaging rate ($19/hr) applies to widefield fluorescence microscope reservations over 12 hours if booked nights or weekends.
Stereomicroscopy
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| Nikon SMZ25 Stereoscope | $28 | $24 | $83 | $138 |
| Nikon SMZ800N Stereoscope | Free | Free | $55 | $110 |
Flow Cytometry - Cell Analyzers
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| BD Fortessa | $50 | $42 | $105 | $160 |
| BD Accuri C6 | $50 | $42 | $105 | $160 |
| Cytek Aurora Spectral Analyzer | $50 | $42 | $105 | $160 |
| Cytek Northern Lights | $50 | $42 | $105 | $160 |
Flow Cytometry - Cell Sorters
| Instrument | Unassisted Hourly Rate | Unassisted After-Hours Hourly Rate | Training Hourly Rate | Assisted Hourly Rate |
|---|---|---|---|---|
| BD FACSAria Fusion | $70 | $60 | $125 | $180 |
| Sony MA900 | $70 | $60 | $125 | $180 |
| Sony SH800 | $70 | $60 | $125 | N/A |
*Longitudinal imaging rate ($19/hr) applies to widefield fluorescence microscope.
These rates are for UT Austin use only. To inquire about our external user rates, please contact the Facility Director.